
Solvation Science Student Challenge (S3C)
Together with RESOLV, explore how solvent molecules influence the selectivity of a reaction!
Take part in RESOLV’s mission to overcome the traditional view of solvent molecules as passive spectators and to consider them as active reactants instead. Use literature research, your knowledge and your creativity to derive a new model that is able to reliably predict the selectivity of a reaction and also takes the influence of the solvent into account.
✅ Challenge completed 🏆 Rewards EUR 3,600 prize pool + partnership programs with RESOLV + exclusive events🌎 Scope International - open to students, PhD students and Postdocs from all over the world
Solvation Science
Most chemical reactions, including many that are central to major industrial and virtually all biological processes, occur in a liquid environment. Beyond the traditional view, it is increasingly recognized that solvents play their own active role in this process.
Gaining a broad understanding of solvation processes offers the opportunity to enable major advances in key new technologies or societal challenges. Whether it's green chemistry and electrochemistry, optimizing industrial processes, avoiding environmental hazards, preventing corrosion, or increasing energy efficiency.
Selectivity
The control of the selectivity (chemo-, regio-, diastereo- or enantioselectivity) of a reaction lies in the focus of many synthetic chemistry studies. To understand the mechanism and the origin of selectivity one needs to distinguish between thermodynamic and kinetic control, which depend on the topography and dynamics along the (free) energy profile of a reaction, characterized by intermediates and relevant transition states.
In synergy with experimental approaches, different computational methods and models were developed in the past to quantitatively predict the outcome of a chemical reaction of interest.
Thermodynamic control can be expected if reaction barriers are comparatively low so that fast crossing under the chosen reaction conditions leads to rapid equilibration. The relative free energies of the products then determine the selectivity.
In contrast, kinetic control is expected when barriers between different reaction channels are high and differ substantially, implying different reaction rates to determine the product spectrum.
The situation becomes much more complicated if multistep reactions occur that pass several barriers of different heights, and when dynamic effects (e.g. caused by the solvent) might prevent crossing even when a system has already climbed the barrier.
Kinetically controlled, e.g. homogeneously catalyzed reactions typically developed in synthetic chemistry can often be well described by applying models like Transition State Theory (see Important Details). In many cases, the selectivity of a chemical reaction is determined in a reaction step that is both rate- and selectivity-determining. To determine the product ratio, one compares the reaction rates that are calculated (via the Eyring equation) from the differences in activation energies that belong to the selectivity-determining transition states. Note, however, that the – usually unknown – “transmission factor” that is related to dynamics along the energy surface can have strong impact on expected outcomes.
Challenge
In one of our recent RESOLV publications, we were dealing with catalyzed reactions, in which the high energy rate-determining step immediately precedes the lower selectivity-determining step.
The problem: Applying the conventional approach described above to predict the selectivity of this reaction, resulted in an unrealistically high E/Z isomerization ratio. The alternatively used Energetic Span Model (see Important Details), on the other hand, predicted the same overall rate for the two reaction channels and therefore resulted in an evenly distributed product ratio. In fact both models don’t match the experimental results; the real values lie in the middle of these two extremes.
One possible explanation could be that solvent effects are not included with sufficient accuracy in the used models. One might assume that in a liquid environment, there should be several collisions on the way from one transition state to the other and therefore the product ratio should be influenced by the solvent.
This raises the key question for our challenge:
How can the influence of the solvent affect selectivity? And thus how might reactions be shifted into a certain direction by a smart and innovative solvent design?
Derive a suitable model that describes reactions with the unusual energy profile mentioned above and that takes the influence of the solvent into account, based on further literature research.
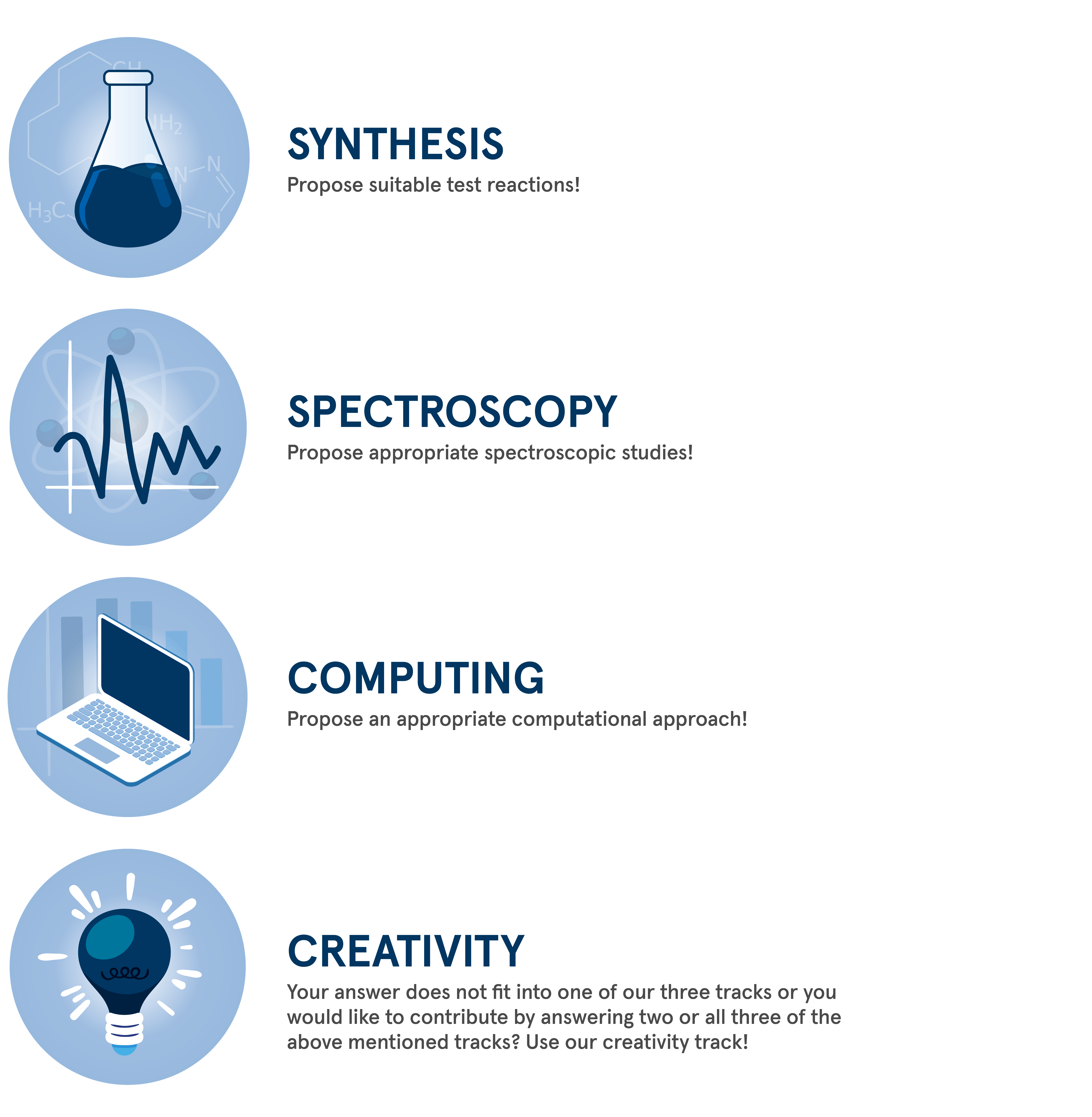
Tracks
Work through one of our track methods to test your model and try to answer the key question above. The different tracks can be approached individually or linked together.
Note: For more information on solution design, be sure to check out the Important Details and Submissions tabs.
Questions or looking for support?
If you want to talk to someone from our team about your approaches, you need more information or questions arise, you are welcome to book a Q&A Call with our project manager Nico or write to us anytime via the chat on this platform to your right.
© 2018- 2026 ekipa GmbH. All rights reserved.