
Benzocaine Innovation Power
Together with Evonik, discover new ways to create and develop a promising specialty chemical which could help us provide better products for our daily lives.
With your ideas, unleash the potential of chemical methods or materials to empower Benzocaine (Et-PABA), and take part in Evonik’s discovery journey in the world of specialty chemicals.
✅ Challenge completed 🏁 Winner Congrats to Roman, PABArotti & Friends and Julius, great effort!🏆 Rewards €10.000 + Evonik as a client or cooperation partner in business or research + Research scholarships
Chemical Background info
To give you an optimal overview of the structure of the Benzocaine (Et-PABA) respectively PABA we are looking for, here is a Chemically correct description.
Background info as a Word-Document
Benzocaine Chemistry and Synthesis
Benzocaine is a crystalline and colorless powder sparingly soluble in water, but it is more soluble in dilute acids and very soluble in ethanol, chloroform and ethyl ether. The melting point of benzocaine is approximately 88-90°C and its density is 1.17 g/cm3. Chemically it is the ethyl ester of para-aminobenzoic acid. Benzocaine is typically prepared by esterification of p-aminobenzoic acid (PABA) with ethanol in the presence of an acid catalyst (Scheme 1) or alternatively by the reduction of the ethyl ester of p-nitrobenzoic acid (Scheme 2).
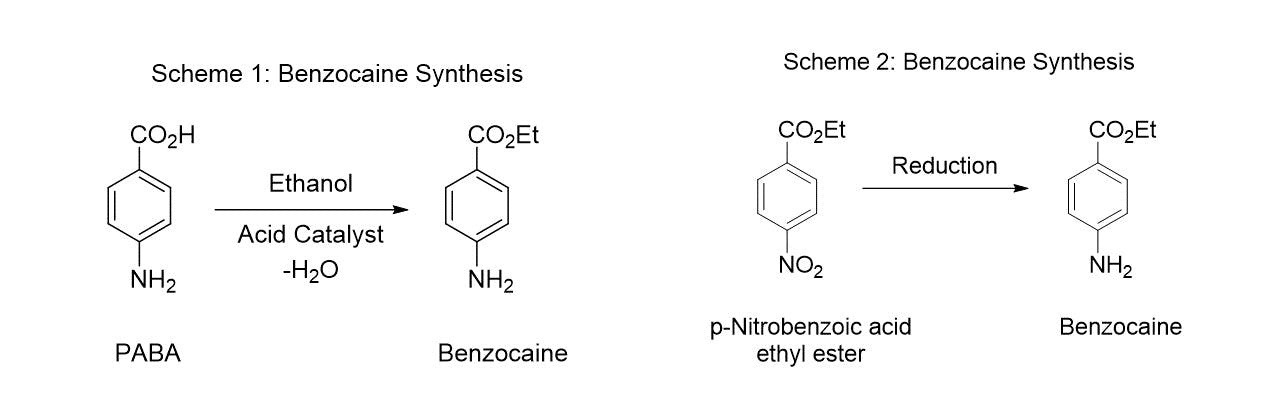
The main raw material used to make PABA is p-nitrobenzoic acid which is typically obtained by the catalytic air/oxygen oxidation of p-nitrotoluene which is obtained by the nitration of toluene. Separation of p-nitrotoluene from the other nitrotoluene isomers can be carried out by re-crystallization in view of the different melting points of the isomers. Other reported techniques include chromatography and adsorption over zeolites (Scheme 3).
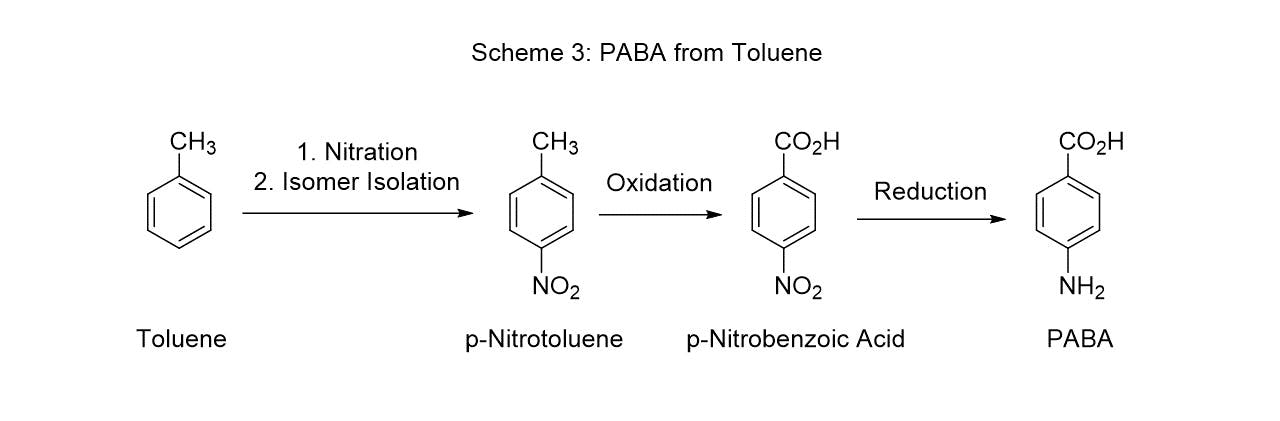
p-Nitrobenzoic acid can alternatively be made by the nitration of polystyrene followed by oxidation. This method improves the para/ortho product ratio due to the steric effect of the polymer chain on the ortho position. (Scheme 4).
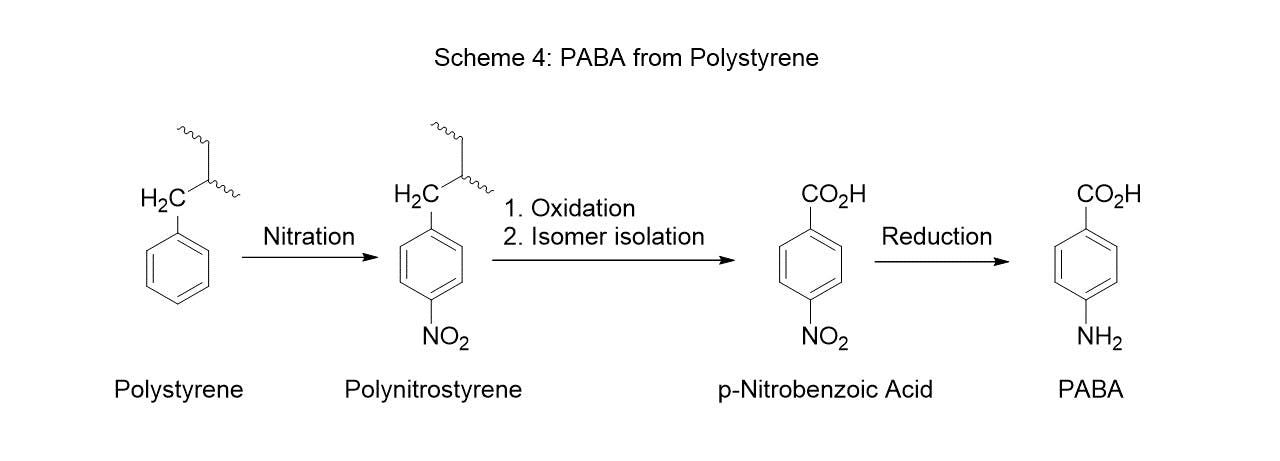
PABA can also be obtained by the Hoffmann degradation reaction of terephthalic acid. Typically, in this process dimethyl terephthalic acid ester is reacted with one equivalent of potassium hydroxide in methanol. The resulting salt is subsequently reacted with ammonia at high temperature (130-140°C) to give potassium terephthalamate which is then reacted with sodium hypochlorite at 50-90°C to give PABA at approximately 70 % yield. More recent improvements to this process can yield PABA with higher efficiency (yield ~ 93 %) (Scheme 5).
Focus of interest: Lower cost Benzocaine and Related Chemical Structures for Technical Applications
General chemical structures analogous to benzocaine which are of interests can be summarized in the general chemical structure shown below (General Structure A) where the substituents X, Y and M can form a saturated or unsaturated hydrocarbon cycle or a saturated or unsaturated heteroatom containing cycle which can be substituted (m > 1) or unsubstituted (m = 0) where the substituent Z can be saturated, unsaturated and substituted or unsubstituted.
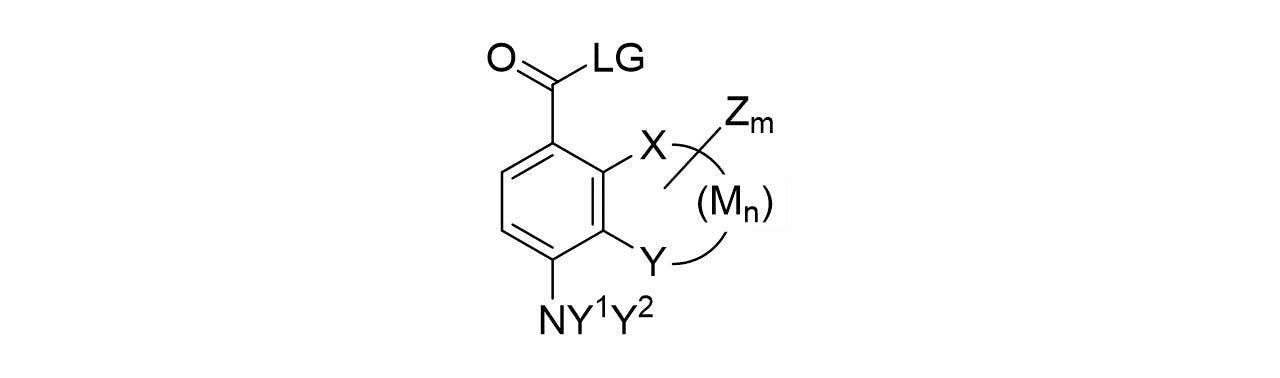
In general structure A, LG represents a leaving group such as -NH2, -OH, -OR1 where R1 = methyl, ethyl, propyl, butyl, pentyl, hexyl and Y1 and Y2 can independently be -H, =O, -R1, =CR22 where R2 = H, methyl, ethyl, propyl, butyl, pentyl, hexyl. Examples of structures of interest include benzocaine where LG = -OR1, X = H, Y = H, R1 = ethyl, n = 0 and m = 0; or alternatives benzocaine-type esters where LG = -OR1 and R1 = methyl, propyl, butyl, pentyl, hexyl; or LG = -OR1 and R1 = methyl, ethyl, propyl, butyl, pentyl, hexyl and X = Y = M = CH and n =2 and m = 0 as for the case of naphthalene analogues of benzocaine-type of compounds or LG = -OR1 and R1 = methyl, ethyl, propyl, butyl, pentyl, hexyl and X = Y = M = CH2 and n = 2 and m = 0 as for the case of a six member cycloaliphatic ring adjacent to benzocaine-type of esters; or heterocyclic compounds such as those obtained when LG = -OR1 and R1 = methyl, ethyl, propyl, butyl, pentyl, hexyl and X = N, Y = NH, n = 1, M = CH and m = 0 for an imidazole ring adjacent to benzocaine-type of esters; or LG = -OR1 and R1 = methyl, ethyl, propyl, butyl, pentyl, hexyl and X = Y = oxygen, M = CH2, n = 2 and m = 0; or LG = -OR1 and R1 = methyl, ethyl, propyl, butyl, pentyl, hexyl and X = Y = oxygen, M = CH, n = 2 and m = 0; and other similar compounds.
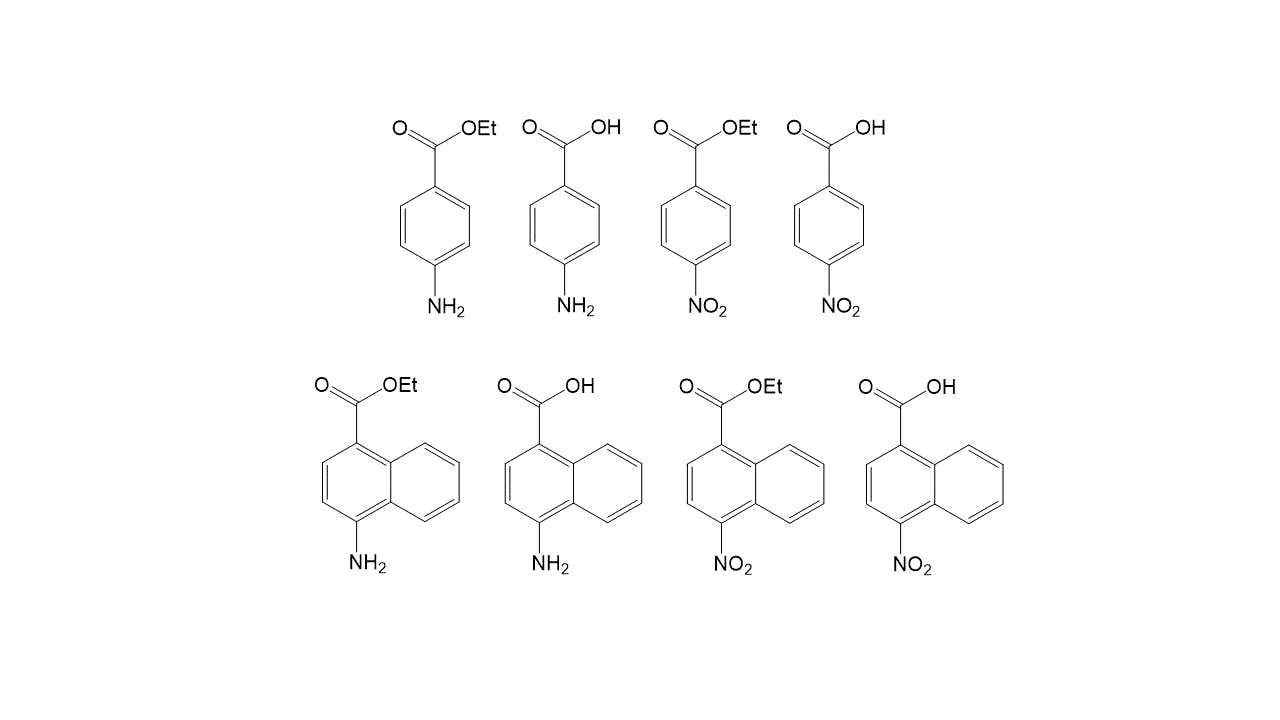
Non-Limiting Representative Examples of Molecules of Interest
© 2018- 2025 ekipa GmbH. All rights reserved.